
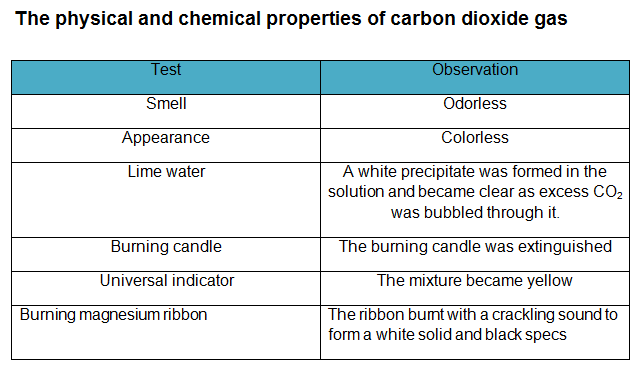
The incomplete combustion carbon or carbon-containing compounds leads to the production of carbon monoxide, CO, in addition to carbon dioxide.
When the supply of oxygen becomes limited, incomplete combustion typically occurs. Intro How to Balance C + O2 CO2 (Carbon + Oxygen gas) Wayne Breslyn 634K subscribers Subscribe 1.1K 125K views 2 years ago In this video we'll balance the equation C + O2 CO2 and.
sulfur will yield sulfur dioxide, SO 2, as one of the products. hydrogen will yield water, H 2O, as one of the products. carbon will yield carbon dioxide, CO 2, as one of the products. Britannica Quiz Pop Quiz: 18 Things to Know About Global Warming At ordinary temperatures, carbon dioxide is quite unreactive above 1,700 C (3,100 F) it partially decomposes into carbon monoxide and oxygen. assuming that the temperature remains constant, the final pressure in the system is atm. Propane, C 3H 8, is a gaseous hydrocarbon that is commonly used as the fuel source in gas grills.Ĭ 3H 8 ( g) + 5 O 2 ( g) → 3 CO 2 ( g) + 4 H 2O ( g)Īs a general rule, combustion of a reactant that contains: the stopcock connecting a 2.76 l bulb containing hydrogen gas at a pressure of 5.68 atm, and a 9.79 l bulb containing carbon dioxide gas at a pressure of 3.84 atm, is opened and the gases are allowed to mix. Many hydrocarbons are used as fuel because their combustion releases very large amounts of heat energy. The products of the complete combustion of hydrocarbons are carbon dioxide and water. 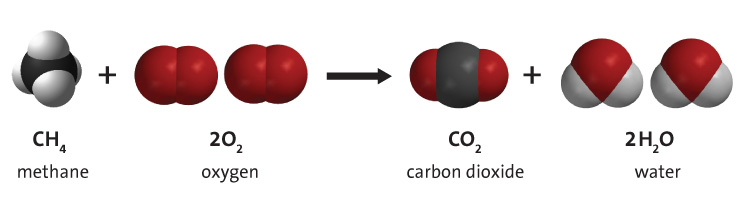
Many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen.






 0 kommentar(er)
0 kommentar(er)
